Background:
In the rituximab era, outcome data from patients (pts) receiving second-line therapy (2L) for diffuse large B-cell lymphoma (DLBCL) are derived mostly from randomized controlled trials or selected cohorts from specialized care centers. As new salvage strategies emerge, there is a need to reevaluate the landscape of 2L DLBCL (treatments and outcomes) in real-world (rw) patient cohorts. We investigated the timing of 2L in a cohort of US pts with DLBCL, the utilization of autologous stem cell transplantation (ASCT) as consolidation, and the overall survival (OS) in these pts.
Methods
We included patients from the Flatiron Health electronic health record (EHR) derived, de-identified database, with a first incident DLBCL diagnosed after January 2011, and with documented 2L therapy receipt (n=524). All pts received ≥4 doses of an anthracycline-based immune-chemotherapy in the frontline therapy (1L) setting and initiated 2L active immune-chemotherapy (anti-CD20 monoclonal antibody monotherapy was excluded) prior to December 2019 to allow for at least 6 m of potential follow-up through May 31 2020. Patients with central nervous system disease at diagnosis were excluded. Real-world overall survival (rwOS) probabilities, indexed to the start of 2L, were estimated in the entire cohort using the Kaplan-Meier method. Hazard ratios (HR) comparing rwOS were estimated using Cox regression models. Baseline covariates included demographic information, DLBCL histologic subtypes, cell of origin (COO) classified by the Hans' algorithm, cytogenetics, and the time interval in months (m) from end of 1L therapy to initiation of 2L therapy. Analyses were also re-indexed to 1 and 3 years post-2L, comparing unadjusted rwOS in pts who had received ASCT on or prior to these timepoints versus those who did not.
Results
In the study sample of 524 pts (57% men), median age at 2L initiation was 68 years (range 59 to 76) and 63% had stage III or IV disease at diagnosis. Initiation of 2L happened within 6 m of 1L completion for a majority of pts, 276/524 (53%), and within 2 months for one quarter, 137/524 (26%); for the rest, 92 pts (18%) initiated between 6-<12 m after 1L completion, 72 pts (14%) between ⩾12-24 m and 84 pts (16%) after 24 m. Consolidation with ASCT was documented for 115 pts (22%) and 17 pts (3%) received chimeric antigen receptor T-cells therapy.
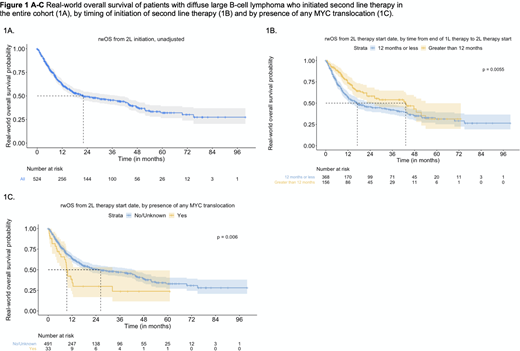
Unadjusted median rwOS in the entire cohort was 22 m [95% CI: 17, 38] (Figure 1A). This varied by interval between 1L completion and 2L initiation; for pts with shorter interval (<12 m) from 1L, median rwOS was 17 m [95% CI: 12, 30], while it was 43 m [95% CI: 22, 54] for those with a ⩾12 m interval (Figure 1B). The presence of MYC translocation alone or in combination with other cytogenetic abnormalities was associated with a shorter unadjusted median rwOS vs. absence of MYC: 9.3 m [95% CI: 8.2, 32, n=33] vs. 26 m [95% CI: 18, 43, n=491] (Figure 1C). In the adjusted model controlling for baseline covariates, older age (⩾ 70) appears to be associated with increased hazard of death (HR: 1.48 [95% CI: 1.14, 1.91]). Unadjusted rwOS analyses at 1 year following start of 2L showed better survival for patients receiving ASCT versus those who did not (HR: 0.40 [0.21, 0.76]). This association was sustained at 3 years (HR: 0.17 [0.04, 0.75].
Conclusion
In a contemporary cohort of rw pts with DLBCL receiving 2L immune-chemotherapy, the majority did not receive ASCT. In this setting, age ⩾70, shorter intervals between 1L completion and 2L initiation, presence of MYC translocations, and no ASCT were factors associated with shorter survival. It may be important to address the implications of these clinical features in the investigation of 2L therapy options for pts with DLBCL.
Sawas:Daiichi Sankyo: Speakers Bureau; Affimed: Research Funding; Roche: Current equity holder in publicly-traded company; Flatiron Health: Current Employment; Gilead: Speakers Bureau; Seattle Genetics: Speakers Bureau. Lau:Flatiron Health, Inc: Current Employment; Roche: Current equity holder in publicly-traded company. Thomson:Flatiron Health, Inc: Current Employment; Roche: Current equity holder in publicly-traded company. Maignan:Flatiron Health, Inc: Current Employment, Current equity holder in publicly-traded company; Roche: Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.